Cognitive Function of the DLPFC in healthy individuals
Generally speaking the DLPFC is believed to be involved in tasks which require working memory, strategizing, planning, social cognition, and also deception.
The DLPFC is involved in a wide range of higher order cognitive processes, it has been found to be involved in generalised intelligence (Barbey, Colom and Grafman 2012),
Figner et al. (2010) found it to be associated with intertemporal decision making, Ochsner Silvers and Buhle (2012) have posited a role for it in the regulation of emotions, particularly with respect of hedonic response through the modulation of the activity of the ventromedial prefrontal cortex, VMPFC, (BA10 BA14 BA25 BA32) while Stienbeis, Bernhardt and Singer (2012) established a correlation between the age-corrected cortical thickness of the left DLPFC and the capacity for the participant children to engage in strategic thinking, the sa,e study found that cortical thickness also correlated with the capacity of these children to moderate impulses.
More recently, Rêgo et al. have argued that while the DLPFC in both hemispheres have an active role in the modulation of pain and personal distress, there is a deviation between the two hemispheres when it comes to feeling the pain of others. The right DLPFC, they found, has a greater role in the “down-regulation of empathic pain through the psychological distancing of the negative stimuli” (Rêgo 2015) than does the left DLPFC.
Such lateralisation has also been found (Perach-Barzilay 2013) with respect to aggression, where suppressed activity in the left DLPFC (where the left DLPFC was inhibited through the use of continuous theta-burst magnetic stimulation), but not the right DLPFC, was correlated with increased reactive aggression, and proactive aggression. While the suppression of the right DLPFC was associated with lower instances of aggression, than the control events.
The Neuroanatomy of the DLPFC.
A difficulty in defining the limits of this area relate to it being defined as a region based upon the functional activity of that area. The DLPFC is associated with executive decision making, in contrast to the more affective processing assumed of the ventrolateral prefrontal cortex VLPFC (BA44 BA45 BA47) that is sometimes included.
In the human brain the DLPFC is a part of the neocortex which covers the frontal lobe. In the anterial-posterial dimension is bounded anteriorly by the anterior-prefrontal cortex (BA10) and the orbitofrontal cortex, OFC, (BA47) at the forefront of the brain. Posterior to the DLPFC is the supplementary motor cortex and the pre-motor cortex (collectively BA6) that lead into the primary motor cortex (BA4).
As this region of the brain is considered along the dorsal-ventral axes there is a certain degree of controversy regarding its extant – different broad and narrow definitions of the DLPFC exist.
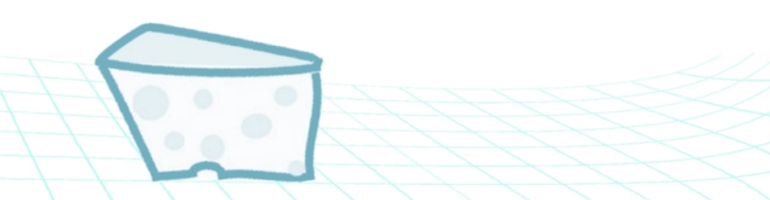
Some definitions of DLPFC include such areas as BA44 and BA45 (which correspond with Broca’s area on the left side of the brain) others include parts of BA10. For the purpose of clarity I will use “DLPFC” to refer to the area “defined as the middle (on the dorsoventral axis) of the line separating the anterior and middle thirds of [the middle frontal gyrus]” (Mylius et al. 2013) which can be approximated by the intersection between BA46 and the lateral parts of areas BA9, see figure 1 to the right.
As part of the frontal lobe of the neocortex the DLPFC has many connections across that surface. In conjunction with the OFC and the anterior cingulate cortex, ACC (BA24 BA32 BA33), the DLPFC is typically taken to be involved in top down thinking and is often characterised as a region of the brain which is deeply involved in “executive function”. As part of this process the ACC is generally taken to be involved in attention, the regulation of emotion, and decision optimisation using reward/pain circuits. Meanwhile the OFC is believed to be involved in inhibitory behaviour. Within this model, the DLPFC is taken to be involved in planning, and complex cognition including social cognition, intensionality, and in combination with the posterior parietal cortex, PPC (BA7) intertemporal discounting.
With respect to the deeper brain structures the DLPFC is connected to the dorsolateral caudate, DLC, of the basal ganglia, then across the striatum, before returning to the DLPFC through the ventral anterior nucleus of the thalamus via the globus pallidus, and through the amygdala via the substantia nigra, which together form the dorsolateral prefrontal-subcortical circuit.
Effect of damage to the region
Through examining and testing Vietnam veterans who had received yrsumstic head injuries that resulted in lesions to the DLPFC, Barbey, Colom and Grafman established that “dlPFC lesions were reliably associated with deficits in general intelligence (g), with noteworthy impairment on measures of working memory and processing speed” (2012).
Etkin et al. (2013) noted that in schizophrenic patients, the activation of the DLPFC was atypical relative to the controls, typically the DLPFCs of schizophrenic patients were less efficient arguing that “patients hyperactivate this region as they strain to keep up at low working memory loads that control subjects can easily handle, and hypoactivate this region at higher working memory loads that exceed patients’ working memory capacity, but not that of controls”.
Chai et al. (2011) found that the activity of DLPFC was decoupled from the activity of the medial prefrontal cortex, MPC, (among health controls these areas were anti-correlated) both in patients who had schizophrenia and also those patients who had bipolar disorder. The MPC is typically active during a person’s ‘resting state’ and consequently when executive control is required, say for solving cognitively complex tasks, it is suppressed while the DLPFC is activated.
Immaturity of the DLPFC which is a result of the slow development that region in children, relative to the development of other brain systems, forms an interesting analogue to lesions in adults. Steinbeis et al. were able to show that intertemporal decision making was correlated not simply with age, but that the variation between children was associated vmPFC–DLPFC coupling.
Tests that assay this region’s function
Given the wide range of functions attributed to this area, there is a comparably large number of tests which are used to establish the effects of the DLPFC.
A typical task to measure the working memory capacity of the brain in general is the N-Back test where the participant is shown a series of images and has to recall whether that image is identical to the image which was shown N steps back, where ‘N’ is an integer, the failure rate is a measure how good the working memory of the participant is, and this can be taxed by increasing the value for ‘N’ thereby increasing the demands on working memory, as used by Masdeuet al. (2014) in their study of patients with Parkinson’s disease.
This has been combined with functional MRI scans to assess the level of activation/inhibition of the DLPFC, in a similar fashion or the use of/distribution of GABA or Glutamate can be measured using proton magnetic resonance spectroscopy in vivo, while the participant engages with cognitive tasks.
Furthermore transcranial magnetic stimulation, TMS, can be used to induce “transient and reversible lesions” (Perach-Barzilay 2012) which have been used to reduce the activation of the DLPFC, while transcranial direct current stimulation, tDCS, can be used to alter the activation patterns of the target area which allows tests to be performed with different degrees of involvement of the DLPFC in a given participant.
Contentious Issues
A great difficulty with the DLPFC is the definition of the area of the cortex that it covers, most descriptions describe an area which is a large portion of the neocortex. It is not always clear whether it is meaningful to refer to this areas as a separable part of the frontal lobe given its co-dependencies.
Typically the region is defined by what it does, however having established a role as the seat of “executive function” in the brain, it is also deeply involved in many other functions of the brain, it is not immediately apparent what role the visual eye fields of BA8 have to do with “working memory” or “executive function” but they are typically included as part of the DLPFC.
But if the DLPFC is well integrated with other circuits so examining the function of it without looking at the other brain structures with which it forms allied circuits of cognition may not be meaningful, or at least making it possible to confuse the consequence of the circuit failure with the role of the DLPFC.
The lateral asymmetry is important too, how does the lack of a Broca area in the non-languaging side of the brain (typically the right) skew structural range of the relevant DLPFC. Even when we consider studies that involve TMS inhibiting the activity of rDLPFC or a lDLPFC we can’t be certain whether a change in behaviour is caused by the dominance of the alternate DLPFC as opposed to the neutralised DLPFC being the seat of the now lost behaviour.
The same concerns emerge in the tDCS studies it is not always certain that the action we take has the effect that we think it has. It is frequently questioned whether the anode/excitation and cathode/suppression relationships are always as clear as they appear. Both these effects, and the effects of age, or head trauma are rarely limited to the area of the brain denoted as the DLPFC so many confounding effects are involved.
Finally even if the effects of these actions/lesions were apparent it is often the case that the brain structure of the individual is as certain as the “5cm rule” would suggest Rusjan et al. have shown, while Seibt et al. (2015) have shown that even where an appropriate area is covered by an electrode, the route of the current is often idiosyncratic to the individual.
Bibliography
Barbey, A. K., Colom, R., & Grafman, J. (2013). Dorsolateral prefrontal contributions to human intelligence. Neuropsychologia, 51(7), 1361-1369.
Chai, X. J., Whitfield-Gabrieli, S., Shinn, A. K., Gabrieli, J. D., Castañón, A. N., McCarthy, J. M., … & Öngür, D. (2011). Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia.Neuropsychopharmacology, 36(10), 2009-2017.
Essex, B. G., Clinton, S. A., Wonderley, L. R., & Zald, D. H. (2012). The impact of the posterior parietal and dorsolateral prefrontal cortices on the optimization of long-term versus immediate value. The Journal of Neuroscience, 32(44), 15403-15413.
Etkin, A., Gyurak, A., & O’Hara, R. (2013). A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues in clinical neuroscience,15(4), 419.
Figner, B., Knoch, D., Johnson, E. J., Krosch, A. R., Lisanby, S. H., Fehr, E., & Weber, E. U. (2010). Lateral prefrontal cortex and self-control in intertemporal choice. Nature neuroscience, 13(5), 538-539.
Masdeu, J., Eisenberg, D., Hegarty, C., Cropp, B., Rubinstein, D., Kohn, P., & Berman, K. (2014). Dorsolateral Prefrontal Cortex Modulation by Caudate Dopamine During a Working Memory Task in Parkinson Disease (P6. 317).Neurology, 82(10 Supplement), P6-317. (POSTER)
Mylius, V., Ayache, S. S., Ahdab, R., Farhat, W. H., Zouari, H. G., Belke, M., … & Lefaucheur, J. P. (2013). Definition of DLPFC and M1 according to anatomical landmarks for navigated brain stimulation: inter-rater reliability, accuracy, and influence of gender and age. Neuroimage, 78, 224-232.
Ochsner, K. N., Silvers, J. A., & Buhle, J. T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences,1251(1), E1-E24.
Perach-Barzilay, N., Tauber, A., Klein, E., Chistyakov, A., Ne’eman, R., & Shamay-Tsoory, S. G. (2013). Asymmetry in the dorsolateral prefrontal cortex and aggressive behavior: a continuous theta-burst magnetic stimulation study.Social neuroscience, 8(2), 178-188.
Rêgo, G. G., Lapenta, O. M., Marques, L. M., Costa, T. L., Leite, J., Carvalho, S., … & Boggio, P. S. (2015). Hemispheric dorsolateral prefrontal cortex lateralization in the regulation of empathy for pain. Neuroscience letters, 594, 12-16.
Rusjan, P. M., Barr, M. S., Farzan, F., Arenovich, T., Maller, J. J., Fitzgerald, P. B., & Daskalakis, Z. J. (2010). Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image‐guided neuronavigation. Human brain mapping,31(11), 1643-1652.
Seibt, O., Brunoni, A. R., Huang, Y., & Bikson, M. (2015). The Pursuit of DLPFC: Non-neuronavigated Methods to Target the Left Dorsolateral Pre-frontal Cortex With Symmetric Bicephalic Transcranial Direct Current Stimulation (tDCS). Brain stimulation.
Snowball, A., Tachtsidis, I., Popescu, T., Thompson, J., Delazer, M., Zamarian, L., … & Kadosh, R. C. (2013). Long-term enhancement of brain function and cognition using cognitive training and brain stimulation. Current Biology, 23(11), 987-992.
Steinbeis, N., Bernhardt, B. C., & Singer, T. (2012). Impulse control and underlying functions of the left DLPFC mediate age-related and age-independent individual differences in strategic social behavior. Neuron, 73(5), 1040-1051.
Steinbeis, N., Haushofer, J., Fehr, E., & Singer, T. (2014). Development of Behavioral Control and Associated vmPFC–DLPFC Connectivity Explains Children’s Increased Resistance to Temptation in Intertemporal Choice.Cerebral Cortex, bhu167.